Pharmacovigilance
Changing regulation and increasing litigation is increasing the focus on pharmacovigilance (PV), general drug safety and clear information for patients.
The NST team will assess your immediate requirements and support in the development of a robust pharmacovigilance reporting strategy. Whether you need full clinical study safety support, ad hoc safety reporting or a more sophisticated analysis of your adverse events, our clinical team can provide a range of trial safety services tailoring our pharmacovigilance offering to the individual needs of our clients. We summarise both safety data and drug benefits in clear, accessible and patient-friendly language. Our Chief Medical Officer is available to provide independent assessment advice.
Clinical study safety support (option A)
The collection and processing of data on serious adverse events (SAE) and adverse events of special interest (AESI) during interventional clinical trials is a fundamental component of pharmacovigilance systems.
In countries where products are undergoing clinical trials, national competent authorities are required to expedite reporting of Individual Case Safety Reports (ICSRs). We offer comprehensive management of cases, from data receipt (data entry, coding, medical assessment and follow-up), through reporting to Competent Authorities, ethics committees/institutional review boards and Investigators as well as coordinating stakeholder communications.
Adverse Events (AEs) Reports: we can meet your AEs handling requirements including interventional and non-interventional studies.
Services include:
- Receipt and triage of initial and follow-up cases SAEs, AESIs, SUSARs, ICSRs, pregnancies, regulatory cases
- Data entry
- MedDRA coding
- Narrative production
- Analysis of Similar Events
- Case quality control
- Medical assessment
- Case closure and locking
- Generation and distribution of follow-up queries
- Expedited case reporting
- Reporting to Competent Authorities, Ethics Committees/Institutional Review Boards and Investigators
- Reconciliation with external data collection partners (CROs, affiliates, partners, etc.)
Safety reporting (option B)
Internationally-harmonized, Development Safety Update Report (DSUR) safety aggregate reports summarise medicinal product clinical trial safety data.
Our reports are aligned with the ICH E2F defined Periodic Safety Update Report (PSUR) format - used for updating the safety record of drugs in their marketing phase, replacing the previous European Union Annual Safety Report and the United States IND Annual Report. Annual DSURs (due 12 months from Development International Birth Dates (DIBDs), can be prepared rapidly and efficiently with the NST team offering the option to coordinate full clinical review and sign-off. The team can also provide ‘published literature’ summary and analysis, medical overview and provide suggestions for company comments.
Our regulatory team are highly experienced in the delivery of 120-day safety update incorporating new safety information that may reasonably affect the statement of contraindications, warnings, precautions and adverse reactions in draft drug labelling. We can also support the preparation of Periodic Adverse Drug Experience Reports (PADER) - largely superseded by Public Benefit-Risk Evaluation Report (PBRER) - which form the basis of EU PSURs: required quarterly for the first 3 years after US approval (annually thereafter).
US Risk Evaluation and Mitigation Strategies (REMS) and EU Risk Management Plans (RMP) are complex documents required for all novel therapies covering the potential risks of the drug’s usage and the precautions to be enforced to manage these risks. The NST team has experience of working on a variety of programmes and can provided broad scope of support. Our appreciation of need to describe clearly the risks associated with medicines in the context of their benefits enables us to express client positions clearly and concisely to foster regulatory agency, healthcare professional and patient understanding.
Our RMPs comply with the EU template (EU-RMP) and include full product information, a safety specification, a pharmacovigilance plan and risk minimization plan, as well as an RMP Summary for Lay Readers.
Analysis of your adverse events (option C)
Burden of Therapy Adverse Events Analysis
Niche provides a means of assessing the overall, grade and adverse event-specific burden of treatment on patients that can deployed at any point during development and can deliver a highly granular assessment of safety. The Burden of Therapy/Toxicity ©™*(BOTh ©™*) analysis is a bespoke service that is statistically tailored to the sponsor safety database. BOTh is highly sensitive and flexible analysis that utilises routinely collected clinical study data and can even be applied both retrospectively and prospectively. BOTh provides:
- a detailed and clinically relevant analyses of safety data
- a clear (visual) representation of the analysis, detailing the comparative burden of any medication or investigation
- a comparative assessment of tolerability and efficacy data
- a means of comparing treatment arms (irrespective of groups size)
- individual patient safety analyses for subgroups or events of interest BOTh Output from analysed clinical trials
A - Examples of Single patient profiles from 2 arms of a study
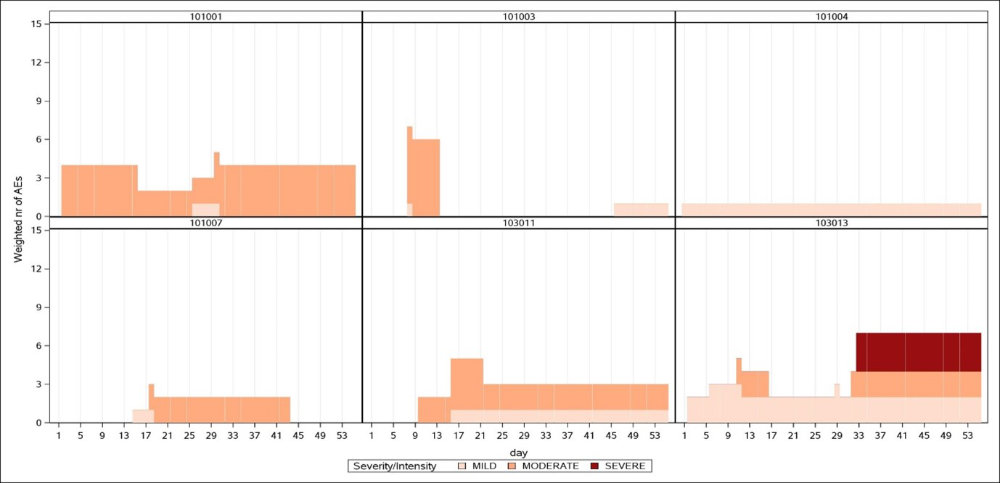
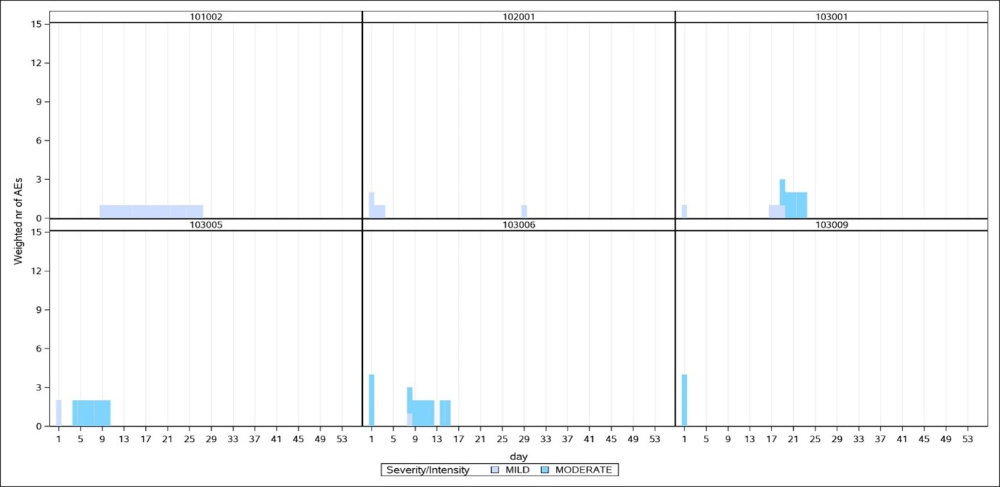
B - Burden of Toxicity by day on 2 arms of the same study
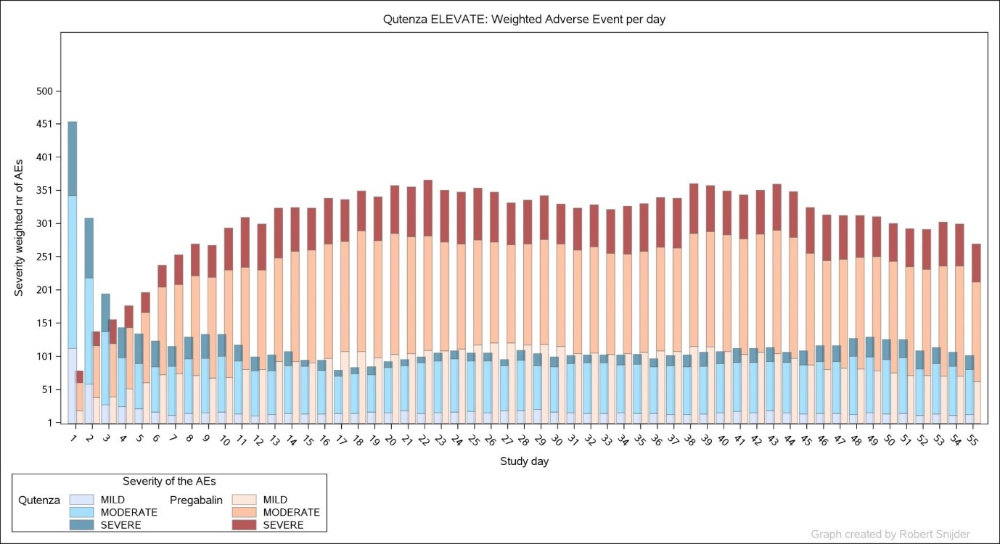
C - The overall burden was 61·2 for the Oral anti-convulsant (Pregabalin) and 23·5 for the topical TRPV1 agonist (Qutenza), (p<0·0001 for between-group comparison)
Published reports of the analysis of several studies by BOTh
1 Ayad K. Abdulahad, Robert J. Snijder, Moeen K. Panni, Faysal K. Riaz,, Andreas J. Karas. (2016)
A novel standard to evaluate the impact of therapeutic agents on patient safety, The BURDEN OF THERAPY™
Contemporary Clinical Trials Communications 4 (2016), 186-191
2 Michael Haas, Ayad Abdul-Ahad, Robert Snijder, et. al.
Implementing a novel method to estimate the "Burden of Therapy" (BOTh) for patients with metastatic pancreatic cancer treated with gemcitabine plus afatinib vs. gemcitabine in the AIO ACCEPT trial.
Abstract #306023, ASCO, 2020, Chicago, May 29-31, 2020
Afatinib plus gemcitabine versus gemcitabine alone as first-line treatment of metastatic pancreatic cancer: The randomised, open-label phase II ACCEPT study of the Arbeitsgemeinschaft Internistische Onkologie with an integrated analysis of the ‘burden of therapy’ method
European Journal of Cancer 146 (2021) 95 -106
 Regulatory writing Regulatory writing
 Strategic communications Strategic communications
 Clinical project management Clinical project management
 Medical education Medical education
 Medical illustration Medical illustration
 Regulatory strategy Regulatory strategy
 Grant applications Grant applications
 Pharmacovigilance Pharmacovigilance
 ILAP Submissions ILAP Submissions |



